(PRLEAP.COM) Chicago, IL – The Cleanroom industry’s smartest, most efficient and regulatory compliant hepa fan filter module will be showcased at the world’s largest HVACR marketplace, The AHR Expo.
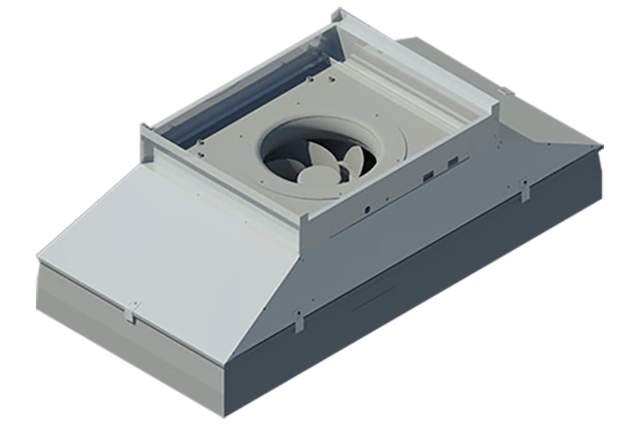
The manufacturers of the SmartHEPA Hepa Fan Filter Module will be on hand to showcase its full potential to anyone in need of this Industry 4.0 ready product, or interesting in becoming an authorized reseller. “We are excited to show off our customer-proven SmartHEPA Fan Filter Module,” said Vern Solomon, President, Concepts To Solutions, SmartHepa manufacturer.
He added, “It is a completely regulatory compliant, energy efficient motor, with modulation that provides feedback for predictive maintenance. Our clients love it! And we are at AHR Chicago booth #3565 for anyone who wants to learn more about why you should choose it.”
“We are excited to be the first authorized North American Reseller of SmartHEPA FFMs,” said Aaron Styles, VP of Operations, ESC Environmental Systems Corporation, an ISO 9001 company specializing in Cleanroom and Critical Environments.
The technology is quickly becoming a must for companies building a new Cleanroom facility or retrofitting their current equipment, and need to ensure regulatory compliance at every step.
For example, SmartHEPA fan filter modules were crucial in the retrofit design at Dalton Pharma.
“We replaced a number of older fan powered Hepa units with the new SmartHEPA in our existing tight plenum spaces. With the extra fan capabilities of the SmartHEPA we are consistently hitting the 90 FPM required for regulatory certification,” said Jim Kanaris, Director of Facilities, Dalton Pharma Services.
This technology will be on display at the 2018 AHR Expo – Chicago, Jan 22nd- 24th 2018 at McCormick Place, Booth 3565. Come see us in person!
About SmartHEPA:
This is the latest patent-pending technology from Concept to Solutions, the company that also manufactures the patented Aluma1 Modular Cleanroom Wall System.
Our projects have been validated to meet GMP, cGMP, FDA, Health Canada & EU standards. We can provide DQ, IQ & OQ documentation and execution and assist with PQ and SOP’s.
Electronics cleanrooms from printed circuit boards to integrated circuits and semiconductor, aerospace and nanotechnology are leading requirements for cleaner manufacturing requirements.